
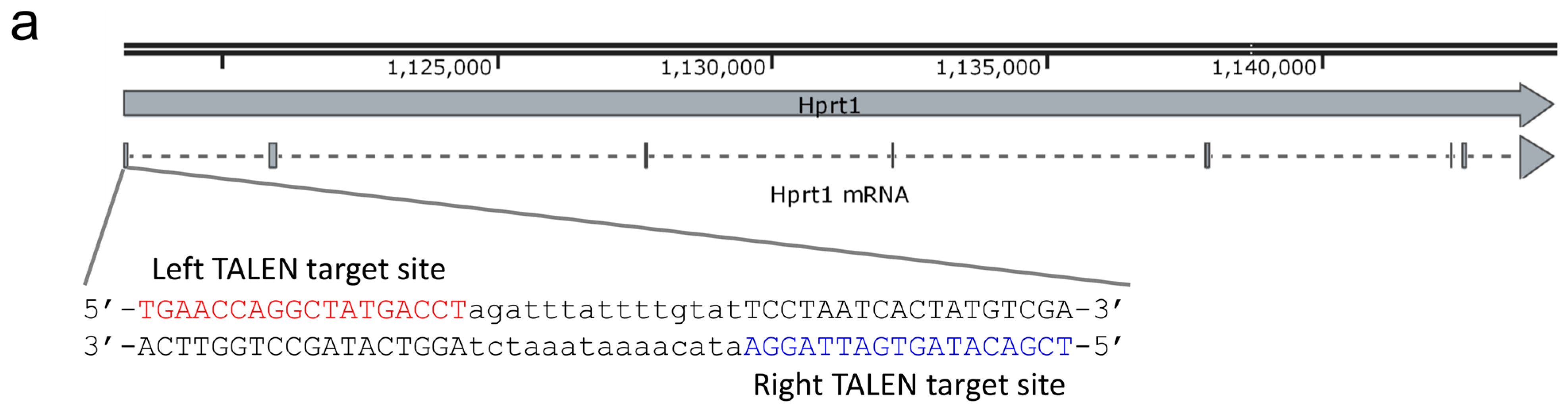
2012), alongside several other species whose numbers are now known to be raised in periodontitis (Kumar et al. 1998 Ximénez-Fyvie, Haffajee and Socransky 2000 Griffen et al. In the case of periodontitis, a dysbiotic community has been characterised by culture-based approaches, DNA-hybridisation and next generation sequencing of 16S rRNA genes, highlighting a group of species associated with disease that was termed the ‘red-complex,’ which included the keystone pathogen Porphyromonas gingivalis, the spirochaete Treponema denticola and the less well-studied Tannerella forsythia (Socransky et al. In the case of the gum diseases gingivitis and periodontitis, alterations in both the host immune response, the subgingival environment and hence the composition of the oral microbial community in this niche are altered in disease.
It is also well established that the relationship between the human host and this bacterial community is key to the homeostasis of health (Darveau 2010 Curtis, Zenobia and Darveau 2011 Hajishengallis, Darveau and Curtis 2012 Ebersole et al. The oral cavity in humans is home to an average of 250 different species per person, with over 700 identified in total, with only around one-third culturable (Thompson et al. With further development, the combination of SpCas9-dual gRNA-corrected DMD patient fibroblasts and transdifferentiation may provide a valuable therapeutic strategy for DMD.Oral microbiology, Tannerella, surface layer, anaerobe, periodontitis, glycobiology INTRODUCTION Restoration of DMD expression at both the mRNA and protein levels was confirmed in the induced myotubes. The edited DMD fibroblasts were transdifferentiated into myotubes by lentiviral-mediated overexpression of a human MYOD transcription factor. More importantly, we further corrected a frameshift mutation in human DMD (exon45del) fibroblasts with SpCas9-dual gRNA ribonucleoproteins. Based on this predictive repair outcome, efficient in-frame deletion of a part of DMD exon 51 was achieved in HEK293T cells with plasmids expressing SpCas9 and dual gRNAs. We first sequenced 32 deletion junctions generated by this editing method and revealed that non-homologous blunt-end joining represents the major indel type. To broaden CRISPR gene editing strategies for DMD treatment, we report the efficient restoration of dystrophin expression in induced myotubes by SpCas9 and dual guide RNAs (gRNAs). CRISPR gene therapy is one promising approach for treatment of Duchenne muscular dystrophy (DMD), which is caused by a large spectrum of mutations in the dystrophin gene.


 0 kommentar(er)
0 kommentar(er)
